A comparative evaluation of the effect of four desensitizing agents (strontium chloride, potassium nitrate, pro-argin, bioactive glass) on dentinal occlusion: An ex-vivo SEM study
Hongal S.1*, Arjun Torwane N.2, Hiremath V.3, Jain M.4
DOI: https://doi.org/10.17511/ijphr.2019.i4.03
1* Sudheer Hongal, Professor & Head, Department of Public Health Dentistry, HKDET’S Dental College and Hospital, Humnabad, Karnataka, India.
2 Nilesh Arjun Torwane, Reader, Department of Public Health Dentistry, Peoples Dental Academy, Bhopal, Madhya Pradesh, India.
3 Vinay Hiremath, Principal & Professor, Department of Oral Pathology, HKDET’S Dental College and Hospital, Humnabad, Karnataka, India.
4 Manish Jain, Professor, SMBT Institute of Dental Sciences and Research, Dhamangaon, Dhamangaon, India.
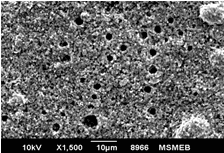
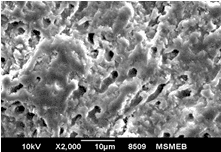
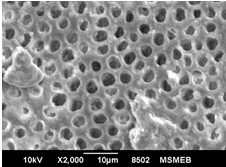
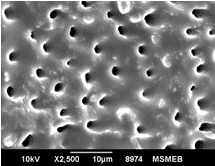
Aim: The aim of this study was to compare and evaluate the effect of four desensitizing agents (Strontium Chloride, Potassium Nitrate, Pro-Argin, Bioactive Glass) on dentinal occlusion. Materials and Methods: Forty freshly extracted mandibular molars were selected. Dentine discs measuring 6x6x3mm were cut off from cervical 1/3rd of molars. The occlusal surface of each dentine disc was abraded with silicon carbide paper for 1 minute and then immersed in 17% Ethylenediaminetetra-acetic acid for 5 minutes. Results: Data were statistically analyzed by one-way Analysis of Variance (ANOVA) and Tukey’s test. The P value for each group was <0.001 showing that highly significant difference existed among the groups. The Bioactive Glass showed maximum amount of dentinal tubule occlusion among all the groups. The least amount of dentinal tubule occlusion was observed in potassium nitrate. Conclusion: It was concluded that tooth brushing with dentifrice containing bioactive glass showed maximum amount of dentine tubule occlusion. Clinical Relevance: Dentinal hypersensitivity is a significant clinical problem which can cause considerable concern for patients. Appropriate and combine use of various clinically effective both synthetic and natural desensitizing agents may considerably help to manage the problem.
Keywords: Dentinal hypersensitivity, Dentinal occlusion, Desensitizing agent
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Professor & Head, Department of Public Health Dentistry, HKDET’S Dental College and Hospital, Humnabad, Karnataka, India. Email: |
Hongal S, Torwane NA, Hiremath V, Jain M. A comparative evaluation of the effect of four desensitizing agents (strontium chloride, potassium nitrate, pro-argin, bioactive glass) on dentinal occlusion: An ex-vivo SEM study. Public Health Rev Int J Public Health Res. 2019;6(4):147-153. Available From https://publichealth.medresearch.in/index.php/ijphr/article/view/113 |


 ©
©