Introduction of pneumococcal vaccine in national immunisation schedule in India: boon or bane?
Nayak R.1*, Kumar Singh A.2, Pragyan Parida S.3, Padhy S.4, Guru Mishra K.5
DOI: https://doi.org/10.17511/ijphr.2019.i6.05
1* Ranjeeta Nayak, Senior Resident, Department of Community Medicine and Family Medicine, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India.
2 Arvind Kumar Singh, Assistant Professor, Department of Community Medicine and Family Medicine, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India.
3 Swayam Pragyan Parida, Associate Professor, Department of Community Medicine and Family Medicine, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India.
4 Sarmistha Padhy, Assistant Professor, Department of Community Medicine, MKCG Medical College, Berhampur, Odisha, India.
5 Kumar Guru Mishra, Junior Resident, Department of Community Medicine and Family Medicine, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India.
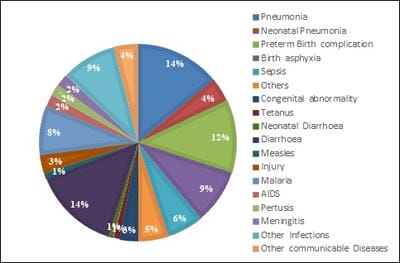
Vaccination is undoubtedly one of the most cost-effective child survival public health measure which are more affordable and accessible to community in preventing diseases. Government of India has undertaken several initiatives to strengthen maternal and child health services in the country and child immunisation being an important part of child healthcare system in India. In recent years, there has been introduction of various newer vaccines. Introduction of pneumococcal vaccine in selected states in year 2017 and plan to scale up in the entire country is the newest change in National Immunization Schedule. This review aims to identify various articles related to pneumococcal infection and vaccine highlighting the burden, serotypic distribution, available vaccines, evidence regarding vaccine safety, efficacy, acceptability and cost effectiveness, in order to provide sufficient understanding on the demand and need of this vaccine in India. Important factors underpinned by this article are though there is a need of vaccination due to severe form of pneumococcal infections and antibiotic resistance due to which many developed countries have included PCV in their regular immunisation programmes, however studies regarding effectiveness of pneumococcal vaccines in developing countries like India, data regarding the burden of pneumococcal infections are not available.
Keywords: Pneumococcal vaccine, Childhood survival, Vaccination
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Senior Resident, Department of Community Medicine and Family Medicine, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India. Email: |
Nayak R, Singh AK, Parida SP, Padhy S, Mishra KG. Introduction of pneumococcal vaccine in national immunisation schedule in India: boon or bane?. Public Health Rev Int J Public Health Res. 2020;6(6):239-245. Available From https://publichealth.medresearch.in/index.php/ijphr/article/view/122 |


 ©
©