Public Perception and Potential Acceptance of COVID-19 Vaccine in India
Bibhabasu Das.1* Padhye A.2
DOI: https://doi.org/10.17511/ijphr.2021.i02.03
1* Bibhabasu Das, Department of Mechanical Engineering, Indian Institute of Technology Madras, Chennai, Tamil Nadu, India.
2 Apurva Padhye, Department of Physics, Indian Institute of Technology Madras, Chennai, Tamil Nadu, India.
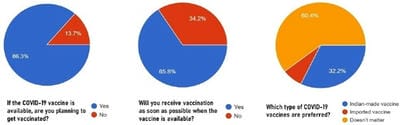
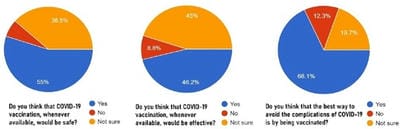
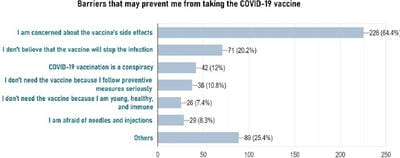
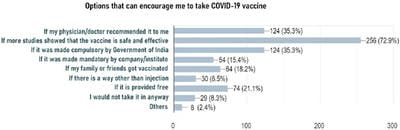
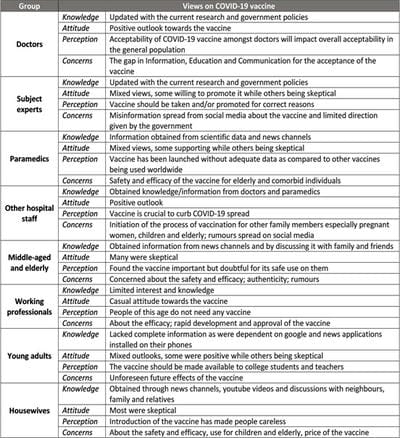
The COVID-19 pandemic, caused by Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, has led to a substantial loss of human life and the global economy, and presents an unprecedented collapse of the public health systems worldwide. The sped-up advancement of the COVID-19 vaccine is an important development. Data from the early trials suggest that the vaccine is safe as well as effective. However, the acceptance of the COVID-19 vaccine among the public depends on various socio-demographic factors. The primary aim of the study is to get a deeper understanding and analysis of the public's perception, information and sentiment towards the COVID-19 vaccine in India.
Keywords: COVID-19, Vaccine Hesitancy, Vaccine Acceptance
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Department of Mechanical Engineering, Indian Institute of Technology Madras, Chennai, Tamil Nadu, India. Email: |
Das B, Padhye A. Public Perception and Potential Acceptance of COVID-19 Vaccine in India. Public Health Rev Int J Public Health Res. 2021;8(2):23-31. Available From https://publichealth.medresearch.in/index.php/ijphr/article/view/156 |


 ©
©