Survey on Incidences of Tuberculosis in Cachar District, Southern Assam, India
Chakraborty A.1, Choudhury P.2*, Bhattacharjee A.3, Dhar (Chanda) D.4
DOI: https://doi.org/10.17511/ijphr.2014.i3.01
1 Apurba Chakraborty, Associate Professor, Department of Zoology, Cachar College, Silchar, Assam, India.
2* Parthankar Choudhury, Ph.D., Associate Professor, Department of Ecology and Environmental Science, Assam University, Silchar, Assam, India.
3 Amitabha Bhattacharjee, Ph. D., Assistant Professor, Department of Microbiology, Assam University, Silchar, Assam, India.
4 Debadatta Dhar (Chanda), MD, Associate Professor, Department of Microbiology, Silchar Medical College and Hospital, Silchar, Assam, India.
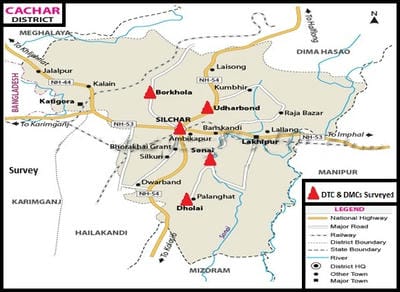
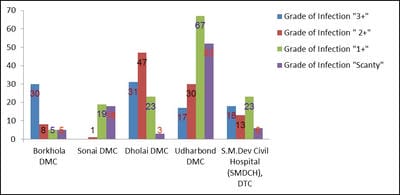
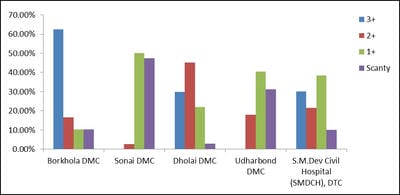
The terms environment and public health are interdependent. Environmental sanitation is the most important parameter that determines the public health of an area. Tuberculosis, a highly contagious disease, caused by the infection of a bacterium, Mycobacterium tuberculosis (a worldwide pandemic) results in more than two million deaths per year. Tuberculosis is most prevalent in underdeveloped countries due to unsanitary living conditions, crowded living quarters and marginalized group of people. Cachar district located at southern part of Assam of North East India has a population of about 17.5 lakhs. A survey on the incidences and severity of tuberculosis was carried out for a period of 19 months (January, 2013 to July, 2014) in the District Tuberculosis Centre (DTC) and Designated Microscopic Centres (DMCs) under RNTCP within the District of Cachar, Assam. The sputum samples of 416 victims were examined to confirm the infection and to ascertain the grades of infection. The results were analyzed according to different age groups, as per grades of infection and on segregation of infection among the males and females. Prevalence and magnitude of infections were found in conformity with the socio-economic status of the victims, most vulnerable age group is between 30-50 years and the infection in males outnumbered the females. The implementation of DOTS, the Stop TB strategy under The Revised National Tuberculosis Control Programme (RNTCP), has improved treatment success rates of tuberculosis and probably led to a decline in the duration of disease.
Keywords: Direct observation therapy, District Tuberculosis Centre, Mycobacterium tuberculosis, Revised National Tuberculosis Control Programme
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Ph.D., Associate Professor, Department of Ecology and Environmental Science, Assam University, Silchar, Assam, India. Email: |
Chakraborty A, Choudhury P, Bhattacharjee A, Dhar (Chanda) D. Survey on Incidences of Tuberculosis in Cachar District, Southern Assam, India. Public Health Rev Int J Public Health Res. 2014;1(3):61-69. Available From https://publichealth.medresearch.in/index.php/ijphr/article/view/8 |


 ©
©